Keep an eye on CRISPR Therapeutics (CRSP), we said on Nov. 2.
“The gene editing stock is starting to take off after a favorable US FDA panel review of the drug exa-cel for sickle-cell disease. Now, should the US FDA approve it on the panel’s vote, we could be looking at the first approved drug using the gene-editing technology,” we noted.
At the time, CRSP traded at around $40. Today, it’s up to $58.58 and could see higher highs, with big catalysts ahead. For one, we’re nearing potential US FDA approval on December 8. Helping, the gene editing stock just beat EPS estimates by 57 cents.
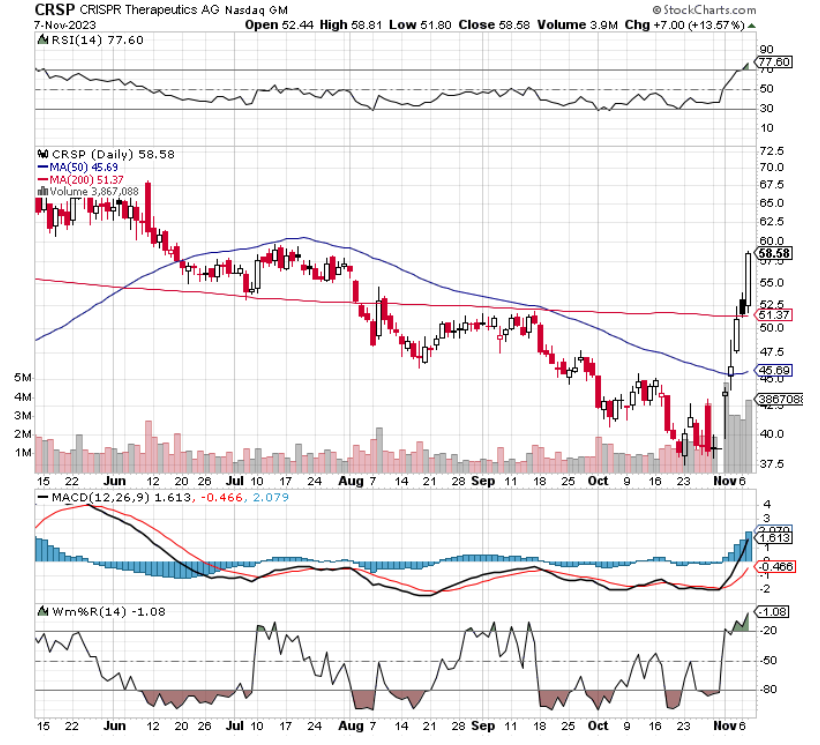

The company also added, “We are excited about the upcoming PDUFA date for exa-cel, which could potentially bring a transformative therapy to patients living with sickle cell disease. If approved, exa-cel would be the first CRISPR-based medicine available to patients in the U.S., highlighting the groundbreaking opportunity of this technology to treat people with serious diseases.
“Additionally, we are excited to initiate clinical trials for our in vivo programs, adding a new pillar to our clinical portfolio. We remain well positioned and well capitalized to bring several transformative medicines for patients suffering from serious diseases.”
Sincerely,
Ian Cooper










Recent Comments